JETRO AnnouncementsApply Now: Regenerative Medicine/Cell and Gene Therapy Startups for Japan Entry Acceleration Program 2025
Sep 08, 2025
JEAP, short for the Japan Entry Acceleration Program, is built on the idea of turning bold visions into real opportunities. The program represents creativity without limits, fresh beginnings in Japan, and the energy to connect globally and grow faster.
With this spirit at its core, JEAP invites pioneering startups in regenerative medicine/cell and gene therapy to join us in shaping the future of healthcare in Japan.
As part of JEAP, selected startups gain access to Japan’s business ecosystem, from tailored mentorship and market insights to direct connections with leading CDMOs/CROs and Pharmaceutical companies, providing the support needed to accelerate entry, collaboration, and growth in one of the world’s most advanced markets.

| Program Name | Japan Entry Acceleration Program 2025 |
|---|---|
| Program Period | September 2025 – March 2026 |
| Application Period | September 8 – October 31 2025 |
| Location | The program will be held mainly online, with the Demo Day taking place in person in Tokyo. |
| Eligibilities |
|
| Capacity | Approximately 5 startups |
| Selection Schedule |
|
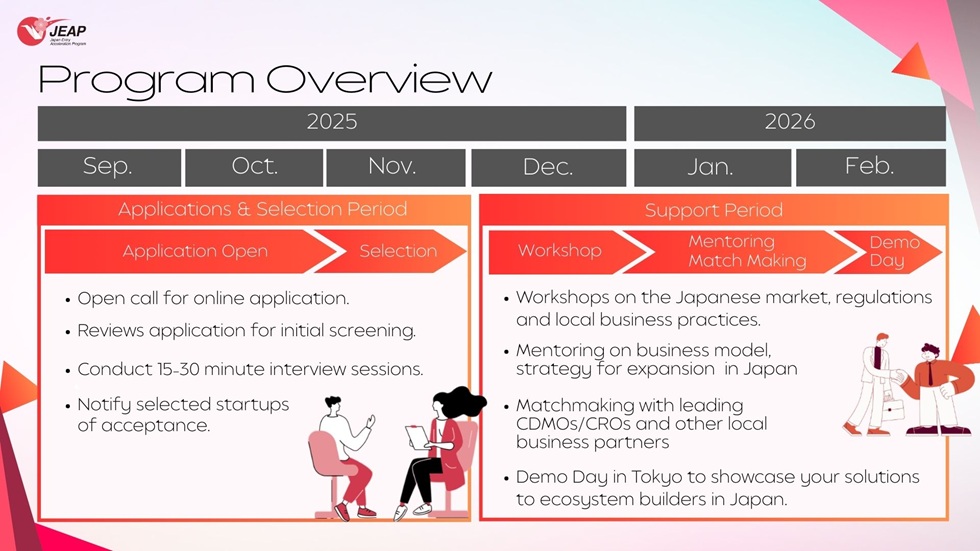
| Program Content |
December 2025:
|
| Participation fee |
Free of charge.
Note: Travel and accommodation expenses will be reimbursed according to JETRO’s internal regulations and budget limits. Expenses beyond the approved scope are not covered. |
| Organizer | JETRO |
How to Apply
Please provide the required information in the application form below.
Application Deadline
Friday, October 31. 2025 23:59
Contact us
JETRO Investment and Alliance Division, Innovation Department(Point of contact: Saito, Ito, Ono)
E-mail: ive@jetro.go.jp Tel: 03-3582-8347
Contact Us
Investing in and collaborating with Japan
We will do our very best to support your business expansion into and within Japan as well as business collaboration with Japanese companies. Please feel free to contact us via the form below for any inquiries.
Inquiry FormJETRO Worldwide
Our network covers over 50 countries worldwide. You can contact us at one of our local offices near you for consultation.
Worldwide Offices