Japan's New Approval System Makes It Easier for Regenerative Medical Therapies to Reach the Market
May 08, 2017
In recent years, the Japanese government has identified a number of priority sectors that it is trying to build up in Japan. These include financial technology, medical devices, aviation/aerospace and Internet of Things (IoT). While progress has been made in all of these sectors, the most dynamic changes have taken place in the area of regenerative medicine. Over the past five years, Japan has positioned itself to be the global leader in regenerative medical therapies, and international life science companies are taking note.

In Japan, regenerative medicine is defined as a cellular or tissue-based product which is cultured or processed from human or animal cells / tissues for the purposes of: reconstruction, restoration or formation of structures and functions of the human body, and prevention or treatment of diseases. Japanese regulators include products transfected into human cells/tissues for the purpose of gene therapy. By 2050, the global market for regenerative medicine is expected to be as much as $345 Billion USD.
Expedited Approval System Sees Regenerative Medicine Drugs Enter Market Faster
In 2014, two new laws governing regenerative medicine went into effect in Japan. The Pharmaceuticals and Medical Devices (PMD) Act creates a new subcategory for “regenerative medicine” and establishes an expedited conditional approval regime for regenerative medicine products. This conditional approval system allows companies to bring regenerative medical therapies to market faster than anywhere else in the world. There are strict safety standards in place and companies are required to provide detailed data to the Ministry of Health, but this new system gives Japan a significant advantage over other countries. Moreover, Japan has a national healthcare system and insurance will cover these new therapies, which gives foreign companies even more incentive to do bring their products to market first in Japan.
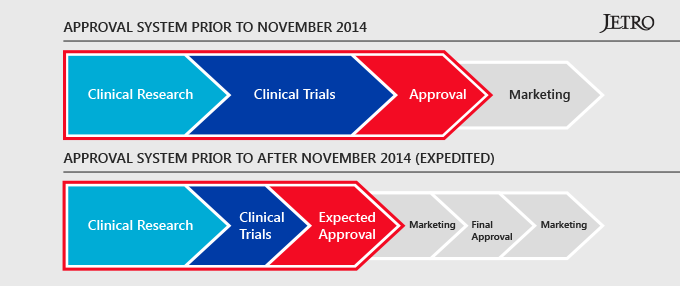
The second law enacted in 2014 was the Act of the Safety of Regenerative Medicine (ASRM), which sets standards for manufacturing regenerative medical products and creates a system for the outsourcing of cell processing. This will allow businesses other than hospitals and research institutes to produce cells for medical use. As a result, a number of major international contract manufacturing organizations (CMOs) have begun the process of expanding into Japan.
Another major development was the establishment of the Japan Agency for Medical Research and Development (AMED). Launched in 2015, AMED was jointly set up by the three government ministries: the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Ministry of Health, Labour and Welfare (MHLW); and the Ministry of Economy, Trade and Industry (METI). AMED is similar to the National Institutes of Health in the USA. It is tasked with leading Japan’s life science R&D efforts and coordinating partnerships between academia and industry. With the creation of AMED, the government is focused on being more strategic and efficient in how it funds medical research.
Minnesota Regenerative Medicine Seminar
To promote the opportunities created by these recent steps, JETRO Chicago organized a seminar in Minnesota on Thursday, February 9. The event was co-organized with the Medical Alley Association, the state’s medical technology trade group, and supported by the Japan America Society of Minnesota, the Minnesota Trade Office, Greater MSP, the U.S. Commercial Service and the Minnesota High Tech Association.
Ralph Inforzato and Robert Corder from JETRO Chicago provided an overview of the recent regulatory reforms and explained how JETRO is working with life science companies to take advantage of opportunities
in Japan. Gil Van Bokkelen, Chairman and CEO of Athersys, also shared his views on the Japanese market and talked about his company’s strategic partnership with Healios, a Japanese company.

Athersys, based in Cleveland, Ohio, is developing MultiStem, a stem cell product for the treatment of diseases in the inflammatory and immune, neurological, and cardiovascular disease areas. Athersys is working with Healios to commercialize MultiStem for the treatment of ischemic stroke in Japan.
Dr. Van Bokkelen explained that the government’s recent moves were motivated by a strong desire to bring cost-effective medical treatments to the Japanese people, as well as to cement their place as a global life science leader. He talked at length about his interactions with representatives from the Ministry of Health and the Pharmaceutical and Medical Device Agency (PMDA). Dr. Van Bokkelen made a strong case for foreign companies doing business in Japan. He noted that Japan is the world’s second largest health care market after the USA and that its national healthcare system makes reimbursement far simpler than in the U.S. or Europe.
Dr. Van Bokkelen emphasized that there is good support from JETRO and the Forum for Innovative Regenerative Medicine (FIRM) for foreign companies interested in Japan.
Learn More About Japan's Regenerative Medicine Market
To learn more about Japan’s regenerative medicine market or how JETRO can help life science companies do business in Japan, contact Ralph Inforzato at ralph_inforzato@jetro.go.jp





