MiRXES Japan Co., Ltd.
MiRXES, a Singaporean company providing life science products and services such as liquid biopsy testing products for early diagnosis and precision medicine, and PCR testing for the novel coronavirus disease, established MiRXES Japan Co., Ltd. in January 2020 in order to expand sales channels in Japan. We interviewed Dr. Li-Foong Yoong, Representative Director of the Japanese subsidiary, about its entry into the Japanese market, business development strategies, and outlook.

- Establishment
- 2020/01
- Destination
- Tokyo
- Biotechnology & Lifescience
-
Singapore
Update : 2021/03
Manufacturer of gastric cancer diagnostic test kits, PCR test kits, etc.
MiRXES is a Singaporean biotech company providing liquid biopsy test products for early detection and precision medicine, RT-PCR testing for the novel coronavirus disease (hereafter, COVID-19), and other life science products and services. Since around 2010, researchers at the National University of Singapore, the company’s founders, have been developing diagnostic testing kits for gastric cancer, using microRNAs in the blood as biomarkers. MicroRNAs are small RNAs contained in body fluids such as blood. Recent studies have shown that the species and quantity of microRNAs in the patient’s blood change due to diseases, including cancer*. MiRXES’s testing kits are promising as a non-invasive diagnostic method for patients enabling rapid diagnosis. The company's technology has been highly regarded by the Agency for Science, Technology and Research (A*STAR). Since 2012 it has participated in A*STAR’s incubation program, receiving financial and other support from the Singapore government.
In 2014, MiRXES Pte Ltd was established in Singapore as an A*STAR spin-off. The company was the first in the world to develop a diagnostic tool for gastric cancer using microRNAs as biomarkers, and succeeded in its clinical application in medical institutions. "Compared to conventional diagnostic methods of early detection of gastric cancer, diagnostic using microRNAs as biomarkers provides more accuracy," says Dr. Li-Foong Yoong, Representative Director of the Japanese subsidiary. Besides gastric cancer, the company develops testing kits for breast cancer and lung cancer and develops and manufactures research reagents and in-vitro diagnostics. In 2020, the company succeeded in mass production of Fortitude Kit, COVID-19 PCR-testing kit. The product has been introduced in more than 80% of all hospitals in Singapore and more than 40 countries, including New Zealand, the U.S., and Latin American countries. The company says that it is filling an application for introduction in Japan.
The company already has offices, R&D labs, manufacturing bases and clinical labs in Singapore, China, and the U.S. In January 2020, it established MiRXES Japan Co., Ltd. in Chiyoda-ku, Tokyo, to expand sales channels in Japan.
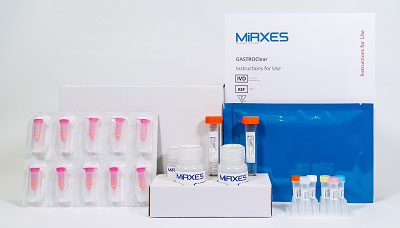
GastroCLEAR, gastric cancer microRNA blood test

ID3EAL microRNA research reagents
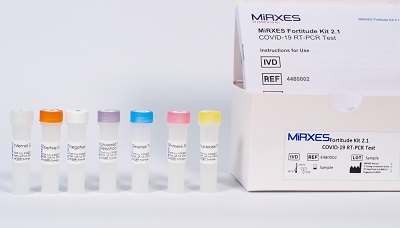
Fortitude Covid-19 PCR kit
Japan is attractive for its market potential and advanced R&D resources
Dr. Yoong explains that the large market size and strong R&D capabilities are the reasons for the company’s interest in the Japanese market. In Japan, the number of cancer patients and deaths are increasing year by year. Japan is known for its high number of gastric cancers among developed countries, which translates into a large market for early cancer detection with unmet demand. Besides gastric cancer, breast cancer and lung cancer are among the “five most common cancers” in Japan, and the importance of their early detection is emphasized. The company is also developing testing kits for breast cancer and lung cancer, anticipating increasing demand for these diagnostics. Japan is also known for its rigorous screening standards for introducing medical devices. The approval in the Japanese market will serve as a strong endorsement for marketing in other Asian countries. This is another reason why we are interested in Japan, says Dr. Yoong.
Dr. Yoong also points to the strong R&D capabilities in cancer diagnostics as another attractiveness of the Japanese market. There are many researchers in microRNAs in Japan, and research on early cancer detection using microRNAs as biomarkers is advancing. Prior to the company's establishment in Japan, researchers at the Singapore headquarters and researchers at the National Cancer Center Japan and the University of Tokyo Hospital were conducting joint research. The Japanese government's initiatives for R&D in the field, such as microRNA-related projects led by the Japan Agency for Medical Research and Development (AMED), also furthered the company’s interest in the Japanese market and establishing a local base.

Dr. Li-Foong Yoong, Representative Director of MiRXES Japan Co
Establishing a local base in Japan and developing business by adapting to the business practices
Before establishing the base, the company had been selling to the Japanese market through local distributors. To expand its sales channels, it established a Japanese subsidiary in January 2020. Dr. Yoong explains that the reason for setting up a local base is the structure of the Japanese market. In Japan, unlike in Singapore, the distribution channel is fragmented by region. Elaborate product explanation and hands-on follow-up for each distributor are crucial. As such, the company needs to have a physical presence in the vicinity of distributors and customers. Also, by establishing a base in Japan, the company has demonstrated its commitment to the Japanese market. "JETRO’s support helped us a great deal in setting up a base in Japan," Dr. Yoong said.
As the company aims to expand its sales channels in Japan, Dr. Yoong says, "Because of the different business practices from Singapore, I think it will be necessary for Japanese business development to have a person who can understand how things work in Japan. Workers’ roles in the workplace are subdivided and multi-layered. The process of application to approval of medical devices is rigorous and requires detailed handling." Dr. Yoong, who assumes the overall responsibility for the Japanese business, was previously a researcher at RIKEN. Working in Japan, she has become familiar with business customs in the Japanese market. Another key for promoting business development in the Japanese market is getting feedback from Japanese researchers on how to tailor products for the Japanese market, such as information about how the company’s products are used in Japanese hospitals and laboratories and product development needs. "Unlike Singapore, Japan has the system of comprehensive medical examination (called Ningen dock) and health checkups," Dr. Yoong says. "Communication with Japanese researchers is vital to know the potential of such a market." As mentioned earlier, researchers at the company's Singapore headquarters had long been conducting joint research with Japanese researchers in microRNAs and others. By continuing research with Japanese researchers, the company believes it can validate demand at medical sites in Japan, leading to developing and supplying products suited to the Japanese market.

microRNA profiling service
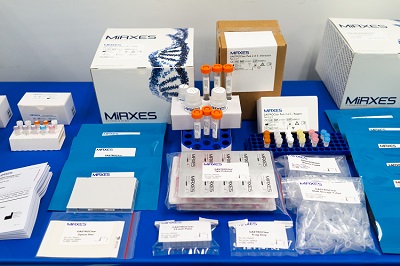
Full range of products for micoRNA research
The research team conducting the study
Continuing to contribute to the R&D of microRNAs in Japan
Eying further business development in Japan beyond expanding gastric cancer test kit sales, the company aims to research and development and commercialize breast cancer and lung cancer products. The company is also working to obtain pharmaceutical approval for selling its RT-PCR test kits in Japan, which have already been deployed worldwide. The company's innovative microRNA technologies and their applications from joint research with domestic researchers will enable simpler and more precise diagnostics for common cancers in Japan, such as gastric cancer. As the company’s Japanese business expands, it will continue to promote microRNAs research and development and extend healthy lifespans in Japan.
(Interview in August 2020)
-
*
Source: Japan Agency for Medical Research and Development, "Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling"
Company history
- 2014
-
Established as a spin-off from the Scientific and Technical Research Agency of Singapore (A* STAR)
- 2019
-
Released the world's first microRNA diagnostic test kit for the early detection of gastric cancer in Singapore and China.
- 2020
-
Succeeded in mass production of Fortitude, a PCR testing kit for COVID-19.
MiRXES Japan Co., Ltd.
- Establishment
-
January 2020
- Business Overview
-
Providing life science products and services such as liquid biopsy testing products for early diagnosis and precision medicine, and PCR testing for COVID-19
- Parent company (group)
-
MiRXES Pte Ltd
- Address
-
6F, Kishimoto Building, 2-2-1 Marunouchi, Chiyoda-ku, Tokyo 100-0005
- URL
Support from JETRO
- Consultation on labor and registration matters
- Information on regulatory matters
- Introduction to service providers
- PR support
Related Industry
Regional Information
Explore More
-

Success Stories
Success Stories are based on interviews conducted with foreign companies and foreign-affiliated companies that have successfully come into the Japanese market.
-

JETRO’s Support
We provide consistent one-stop service for establishing a base or expanding business in Japan.
-

Setting up Business
You can find information on overall and detailed steps, cost estimation, an overview of the laws, regulations and procedures related to setting up business, and more.
Contact Us
Investing in Japan
We will do our very best to support your business expansion into and within Japan. Please feel free to contact us via the form below for any inquiries.
Inquiry FormJETRO Worldwide
Our network covers over 50 countries worldwide. You can contact us at one of our local offices near you for consultation.
Worldwide Offices
























