Meet Japanese Companies with Quality
Expanding the Horizons of Biologics Gene Techno Science Co., Ltd. IP
Website: Gene Techno Science Co., Ltd.![]()
Category: Others
Gene Techno Science brings essential know-how and a fabless structure to the development of biologics and biosimilar pharmaceuticals
Gene Techno Science Co., Ltd. develops biologics and biosimilar pharmaceuticals using a “fabless” development structure that gives it the speed and flexibility to compete with big pharmaceutical companies. Meanwhile, the sheer difficulty of developing manufacturing processes for biosimilars gives the company a treasure-trove of know-how that is hard to match.
As patents on the world’s longstanding stock of chemical-based small molecular drugs continue to lapse, many pharmaceutical companies are turning to biologics—large-molecule drugs developed from biological sources—in search of new intellectual property.
“The world is shifting more and more to biologics,” says Masanari Kawaminami, president of Gene Techno Science. The company was formed in 2001 by scientists at Hokkaido University to develop biologics and what are called “biosimilar” pharmaceuticals: generic treatments based on lapsed-patent biologics.
Comprehensive Biologic & Biosimilar Know-how
Gene Techno Science has had successes both in its biologic and biosimilar lines. In 2007, it developed, patented and out-licensed an anti-α9 integrin antibody in Japan, and is now looking for partners to help take the product overseas.
The company also conducted joint research with Fuji Pharma Co., Ltd. to bring the first filgrastim biosimilar (used to treat low blood neutrophils) to the Japanese market in May 2013. The success of the filgrastim biosimilar is supporting the company as it expands alliances and carries out basic research for biosimilars in the areas of kidney diseases, autoimmune disorders, oncology and ophthalmology, and it’s looking to expand into regenerative medicine as well.
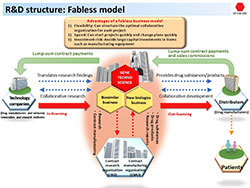
Fabless Development for Speed & Flexibility
The extreme difficulty of development means simply establishing the manufacturing process for a biosimilar creates a stock of know-how unique to the company. “There are only about two Japanese pharmaceutical manufacturers that make their own biologics,” Kawaminami explains. “There are few companies that have the manufacturing know-how.”
However, rather than engage in manufacturing itself, Gene Techno Science partners with various organizations—including technology companies, distributors, contract research organizations and contract manufacturing organizations—to completely outsource fabrication. Kawaminami says this fabless structure gives his organization speed and flexibility that offer advantages over larger pharmaceutical manufacturers.

Gene Techno Science Co., Ltd. President Masanari Kawaminami.
Biosimilars for Local Markets
Kawaminami believes Gene Techno Science has a greater mission to fulfill beyond mere financial success: “At the very least, Japan should be able to make its own biosimilars,” he says.
In addition to its home market, the company has an eye on North America, Europe, South America and the greater Asian sphere, seeking partners that will assist in conducting clinical development, acquiring regulatory approval and conducting distribution and sales in each local market. Eschewing global pharmaceutical giants, the company hopes to find strong local enterprises to help establish a solid base in each area, having just partnered with a Chinese company in 2016 to expand its biosimilar business in that region.
With a large number of biologics slated to go off-patent by 2020, Gene Techno Science’s potential promises only to grow.
Based on interview in January 2017
Website: Gene Techno Science Co., Ltd.![]()